Toronto / Boston
Canada / USA
647-214-0185
Your Custom Text Here
Your Custom Text Here
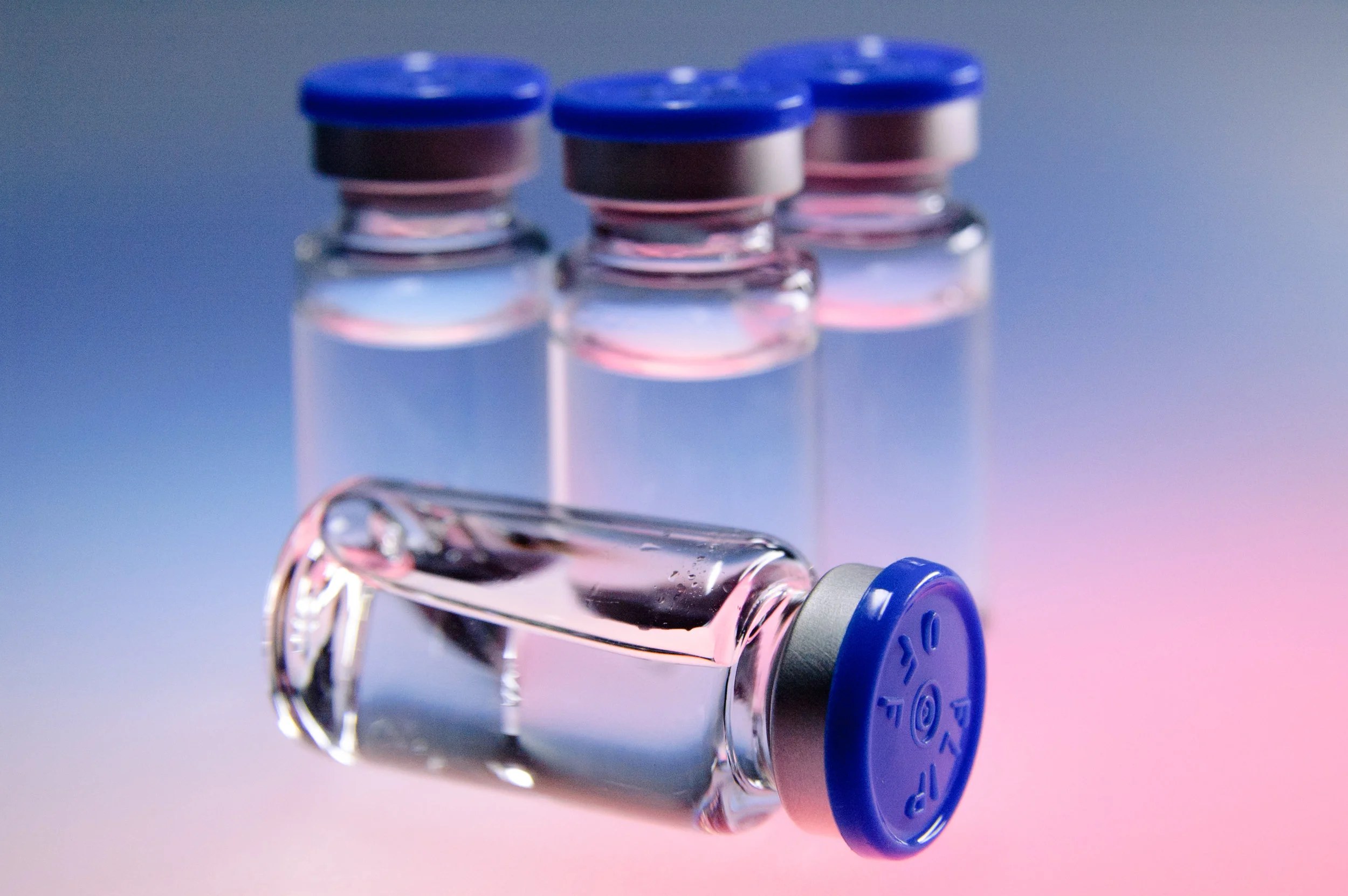
Your product's sterility assurance is one of its most important quality attributes. We offer support services in all aspects of sterility assurance including process design, aseptic reliability assessments, media fill expertise, regulatory dossier authoring and process improvements.
Our technical expertise spans cycle development to validation for steam sterilization, dry heat sterilization and depyrogenation, irradiation and vapourized hydrogen peroxide sanitization.
Your company's validation program is front and centre in regulatory inspections and license applications. We can help you in design of new programs or revitalizing a legacy program. Our experience spans all validation types including laboratory methods, equipment, facility, sterilization, sanitization, disinfection, cleaning, depyrognation and process.
Quality risk management is a vital element of understanding your product or manufacturing process. Our extensive experience with a variety of risk management tools can be applied to proactively identify opportunities to improve quality, reduce validation requirements, improve sterility assurance or guide product development leveraging Quality by Design.
It is now an expectation that you improve the microbiological control of your upstream manufacturing processes, especially for biologically derived products. We can help you proactively reduce risks in your upstream low bioburden processes by leveraging risk management principles and implementing new technology.
Leverage our years of experience in Quality Assurance at leading pharmaceutical and biotechnology companies. Let us help you develop your pharmaceutical quality system from the ground up or improve your existing systems. We also provide auditing services from mock pre-approval inspections, general GMP to supplier / partner audits. Let us help you prepare for your next regulatory inspection by helping you focus on potential areas of interest from the Inspectors, preparing information packages and coaching presenters.
Education of staff in a pharmaceutical manufacturing environment is an important backbone of any operation. Teaching those that touch the process the reasons behind the elements of your procedures will reduce errors and improve quality. We can tailor and deliver training sessions to your facility in basic microbiology, sterilization, depyrogenation, cleaning and disinfection or any other aseptic processing topic.